pharma
21 CFR Part 11 Compliance SCADA system
Application Overview
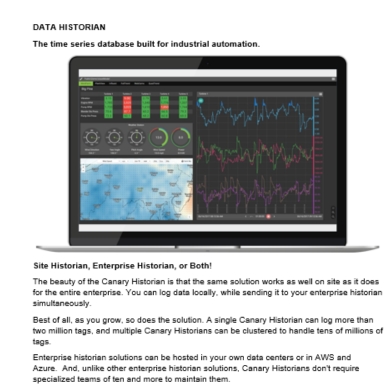
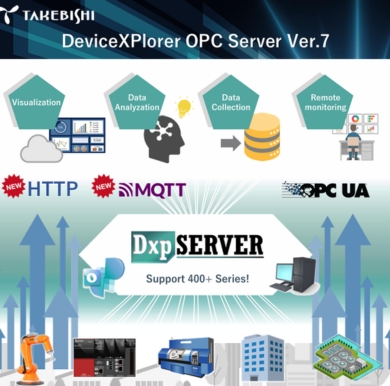
Our SCADA provides robust automation software solutions that support compliance with FDA 21 CFR Part 11, which governs electronic records and electronic signatures. It is ideal for applications in regulated industries such as pharmaceuticals, biotech, food and beverage, and life sciences. HMI/SCADA systems allow for secure data logging, electronic signatures, audit trails, and secure user authentication, reports.
Graphics Screen Layout
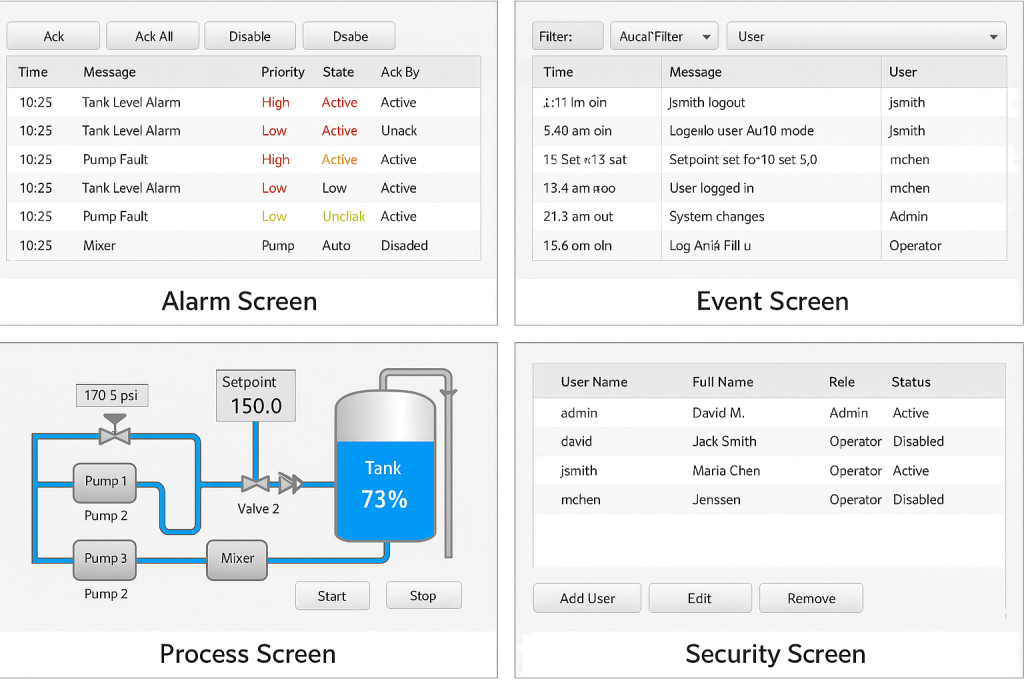
- Dashboard Summary Screen – showing KPIs, alarms, and overall system status.
- Equipment Monitoring Screen – real-time visualization of machines and sensors.
- Alarm & Event Viewer – logs alarms with timestamp, acknowledgment, and user traceability.
- Audit Trail Viewer – detailed logs for changes made in system settings or values.
- Electronic Signature Input – user sign-off and comments with secured role-based access.
KEY FEATURES SUPPORTING 21 CFR PART 11
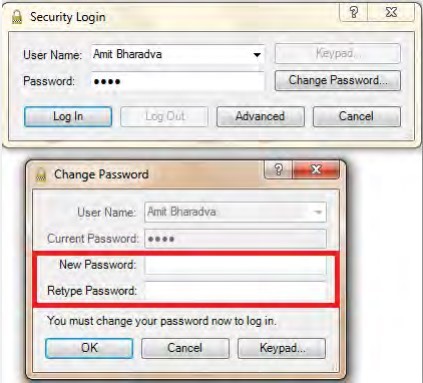
- Secure user authentication and role-based access control
- Encrypted data logging and archiving
- Electronic signatures for process changes
- Tamper-proof audit trails with timestamps
- Centralized user management and password policies
- OPC UA/DA (Client & Server)
- Modbus TCP/IP and RTU
- BACnet/IP, SNMP, MQTT, Web services
- SQL and ODBC database integration